
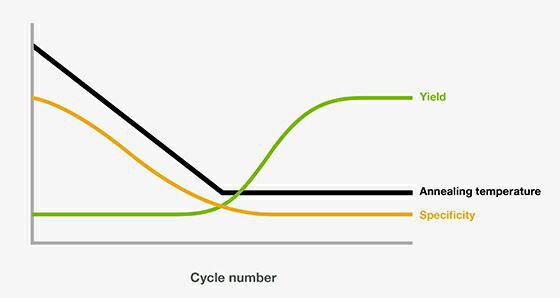
By this point, ideally, the number of perfect match priming sites from early cycle amplicons helps compete on a number of copies basis against the un- (or at least, less-) amplified spurious sites. Thus, a single copy target gets preferentially (but very weakly) amplified in the first cycle, slightly less preferentially (but a bit more strongly) amplified in the second cycle, and so on until “optimal” Tm is reached. What happens – thinking back to our Boltzmann curve – is that at temperatures well above ideal annealing, only a very small fraction of primers will have low enough thermal energy to bind, and such binding will be biased towards the perfect match sites. Over the next 10 to 20 cycles, the annealing temperature is decreased by ~0.5⁰ - 1.0⁰C per cycle. In this, rather than use the predicted optimal primer-annealing temperature, one starts off the initial PCR cycles with annealing step temperatures well above that often, as much as 10⁰C over predicted Tm. One way to enhance specificity in this scenario is by what is called Touchdown PCR. While these will be thermodynamically unfavored compared to the perfect match, annealing occurs along a Boltzmann distribution curve: put more simply, at least some annealing will occur at less-than-perfect binding sites and if your primers are less than ideal from a “uniqueness” standpoint, this can be a significant proportion. In reality, unavoidable constraints on amplicon design based on minimum and maximum product sizes for efficient amplification, limited areas of sufficient genetic conservation pressure to serve as reliable primer sites, target genome size and GC content, and other factors can sometime leave an assay stuck with primers that have more than passing homology to unwanted secondary binding sites. Ideally, one would design primers that have absolutely no significant similarity to template sequence elements other than the intended site. Part of the key to this is the “uniqueness” of each primer sequence, or how many near-match binding sites there exist in a template population as compared to intended annealing site(s). Specificity in a PCR reaction relates mechanistically to ensuring that the forward and reverse primers anneal productively at their intended priming sites, and not at a bunch of other unwanted loci. In this month’s episode of The Primer, we’re going to peek under the hood at some of the alterations that can be done to the underlying core process, how these impact product formation, and why or where you might find these applied. Aspects like making the PCR into a real-time assay involve complications with adding means for ongoing product detection, but those don’t change the mechanics of how the product is formed. In a lot of assays, that’s really about all there is to it, too. Two primers, some buffer, and thermocycling including denaturation, annealing, and extension steps and you’re good to go. Few, if any, readers of this column won’t have at least a basic appreciation for the process (similar to that outlined in this space in the May 2013 edition, ).
By now, the polymerase chain reaction (PCR) is no longer the new big thing in molecular biology and diagnostics it’s not only taken for granted as the underpinning method behind a vast number of clinical tests, it’s the sort of thing taught in high school biology.


 0 kommentar(er)
0 kommentar(er)
